The pharmaceutical industry is one of the most regulated industries, and compliance with current Good Manufacturing Practices (cGMP) is not just a requirement of law, but also a cornerstone of quality assurance, safety and quality of operations. As a leading manufacturer of pharmaceutical process equipment, Stalwart International understands the importance of designing and delivering cGMP-compliant systems that uphold the highest industry standards.
Why cGMP Compliance Matters in Pharmaceutical Equipment
1. Prevents Contamination Dangers
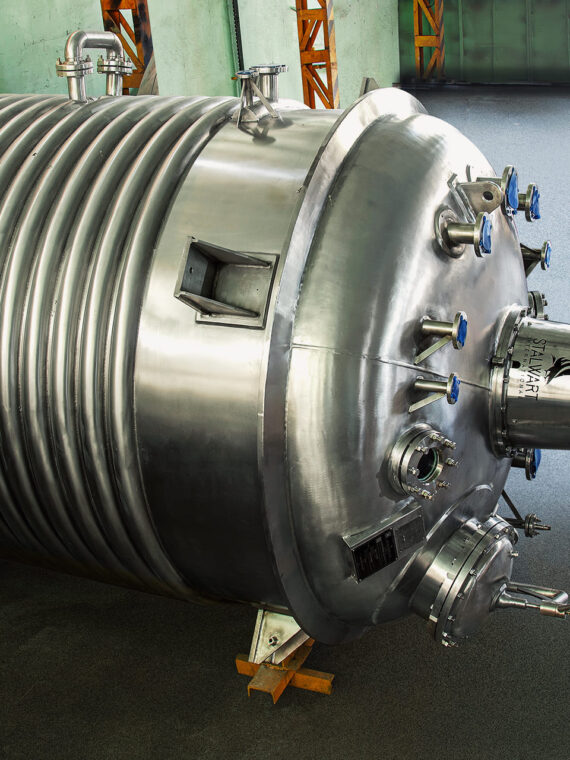
One of the greatest risks to the integrity of a pharmaceutical product is contamination. cGMP requirements are aimed at curbing such risks through the establishment of high standards of cleanliness and hygiene. Stalwart’s equipment, including pharmaceutical reactors, filter presses, and heat exchangers, is engineered with high-grade stainless steel (SS316/SS316L) and smooth surface finishes that reduce the chance of bacterial growth, product buildup, or corrosion.
Others like the CIP (Clean-in-Place) and the SIP (Sterilize-in-Place) are incorporated, and this makes cleaning and sterilization complete and repeatable. This significantly reduces the risk of cross-contamination between batches, ensuring drug safety.
2. Complies with Regulation
The pharmaceutical products should be produced with the help of cGMP-compliant equipment required by such regulatory agencies as the FDA, WHO, and EMEA. When they do not achieve these standards, recalls, penalties or closure may be obtained.
Stalwart has compliance-designed process equipment. Our systems are constructed to the ASME standards, the FDA regulations and the ISO 9001:2015 certifications. This assures the pharmaceutical producer to pass the inspection, audits and market approvals with speed.
In addition, documentation that accompanies all our equipment delivery is cGMP compatible, such as equipment validation procedures (IQ/OQ/PQ) and traceability, as well as electronic records.
3. Enhances Process Efficiency
Pharmaceutical manufacturing efficiency does not imply that you are fast but rather repeatable, replicable, and scalable. cGMP-compliant equipment streamlines the manufacturing process by bringing in automation, control systems, and precision engineering.
Our pharmaceutical-grade heat exchangers and condensers, for example, are designed to deliver precise temperature control, the cornerstone of reaction stability and product yield.
4. Improves Product Quality
Consistency in product quality is a must in the pharmaceutical industry. cGMP-compliant equipment allows for consistent mixing, controlled reaction, and effective separation of materials, which all amount to quality product.
With features like jacketed vessels for uniform heating/cooling, baffle design for mixing at its optimum, and online monitoring systems in real time, Stalwart’s pharmaceutical processing equipment and reactors are built to deliver reproducible results. That means greater product efficacy, reduced batch rejection, and customer delight.
5. Enables Scalable Growth
The companies that deal with drugs are growing and developing new formulations and thus need a set of equipment that can be scaled up to suit their processes. Stalwart’s cGMP-capable systems are built to scale, from laboratory-scale R&D configurations to full-scale commercial production lines.
Through modular designs, custom engineering, and standard documentation, scaling up is easier, quicker, and more cost-efficient. From making small molecule medications, vaccines, or biologics, our equipment stands ready to scale with you.
You May Also Like : Importance of cGMP in the Pharmaceutical Industry
Final Thoughts
cGMP-compliant process equipment is more than a regulatory requirement—it’s a foundation for quality, safety, and efficiency in pharmaceutical manufacturing. It minimizes contamination risks, ensures consistent product quality, and supports regulatory compliance.
At Stalwart International, one of the leading cGMP equipment manufacturers in India, we provide high-performance pharmaceutical equipment including reactors, heat exchangers, condensers, pressure vessels, filter presses, and mixing tanks. With more than three decades of experience and global reach, we’re committed to boosting your facility’s productivity and compliance.
Looking to upgrade your pharma setup? Contact Stalwart International for reliable, cGMP-grade equipment solutions.
FAQs
1. What does cGMP stand for in pharmaceuticals?
cGMP stands for Current Good Manufacturing Practices. It refers to the latest standards and regulatory guidelines set by the FDA to ensure pharmaceutical products are consistently produced and controlled according to quality standards. The “current” in cGMP emphasizes the need for companies to use up-to-date systems, technologies, and practices.
2. Why is cGMP compliance important for pharmaceutical equipment?
cGMP-compliant equipment is essential because it reduces contamination risk, improves process consistency, and ensures batch-to-batch quality. This leads to safer and more effective drug production, meeting both regulatory and patient safety requirements.
3. What is the role of process equipment in cGMP compliance?
Process equipment plays a vital role by ensuring controlled environments, maintaining proper documentation, supporting cleanability and sterilization, and preventing cross-contamination — all of which are cGMP essentials in pharmaceutical manufacturing.
4. How does cGMP-compliant equipment improve drug safety?
It enhances safety by using validated materials, supporting automated cleaning-in-place (CIP) and sterilization-in-place (SIP) systems, reducing human error, and ensuring traceability in the entire manufacturing process.
5. How does cGMP affect drug manufacturing efficiency?
With cGMP-compliant equipment, processes become faster, more accurate, and less prone to failure. Automated systems and real-time monitoring minimize downtime and reduce manual intervention, thus improving overall manufacturing efficiency.
6. What is the difference between GMP and cGMP?
GMP stands for Good Manufacturing Practices, a baseline quality standard. cGMP adds “current,” requiring companies to continuously update their practices and technology to align with evolving regulations and industry best practices.
7. What is 21 CFR Part 210/211 in relation to cGMP?
21 CFR Parts 210 and 211 are FDA regulations that outline cGMP requirements for manufacturing, processing, packaging, and holding of drugs. They cover equipment design, personnel hygiene, recordkeeping, quality control, and more.
8. Who is responsible for ensuring cGMP compliance in pharma manufacturing?
Both the pharmaceutical manufacturer and equipment suppliers are responsible. Manufacturers must verify that all equipment is cGMP-compliant, while suppliers (like Stalwart International) must design and deliver systems that meet these standards.
9. How can I tell if process equipment is cGMP-compliant?
Look for equipment that offers full documentation (DQ, IQ, OQ, PQ), is easy to clean, supports automation, and is built using pharma-grade materials. Reputable manufacturers will also provide validation support and comply with FDA and international guidelines.
10. Does cGMP compliance also apply to cleanroom equipment and utilities?
Yes, cGMP standards apply to all critical components of drug manufacturing — including cleanrooms, HVAC systems, water systems, and utilities. These must also meet hygiene, monitoring, and material standards to ensure overall compliance.