When it comes to pharmaceutical manufacturing, there is no room to be inaccurate and inconsistent. The core of this complicated process is a very important part, namely, the pharmaceutical reactor. Active Pharmaceutical Ingredients (APIs), intermediates and specialty compounds are all manufactured in pharmaceutical reactors, and both the design and scale-up of these reactors have a direct influence on product quality, safety and regulatory compliance.
Whether in small-scale laboratory experiments or the big scale commercial production, reactor design is a crucial element in getting the best outcomes. This blog is about what you need to know about pharmaceutical reactor design, the pitfalls of scale-up up and some understanding of how a manufacturer of process equipment, such as Stalwart International, can provide reactor systems with industry-compliant performance.
What Is a Pharmaceutical Reactor?
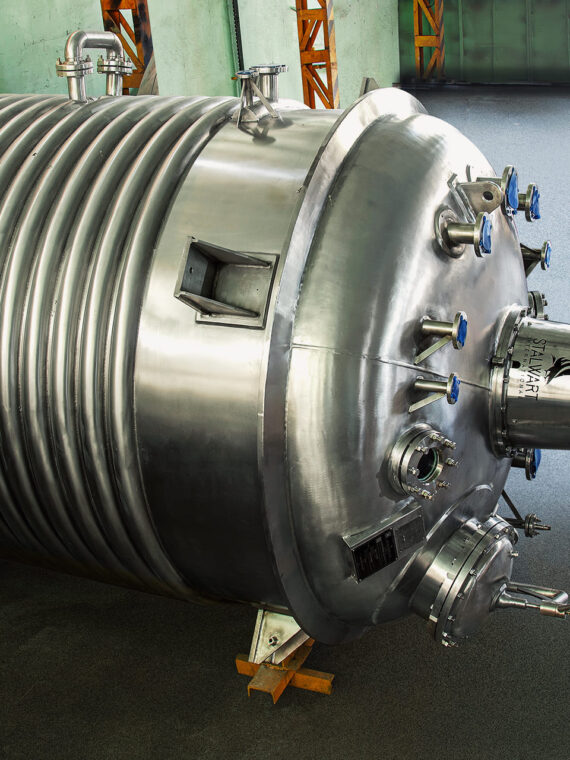
A pharmaceutical reactor can be described as a special vessel that is operated to provide a chemical reaction with a certain temperature, pressure and mixing conditions. These reactors are inseparable in the synthesis of drug compounds, particularly in the APIs and intermediates manufacturing.
The pharmaceutical reactors should be able to match the high standards in the industry to guarantee purity, reproducibility, and safety of the products. They are often called upon to be cGMP, clean-in-place (CIP) or steam-in-place (SIP) compliant and constructed out of corrosion-resistant alloy SS316L or Hastelloy.
Types of Reactors Used in Pharmaceutical Manufacturing
Various processes have varied reactors. The most prevalent type of reactors used in the pharmaceutical environment is as follows:
1. Batch Reactors
Pharmaceutical labs and production units widely use them because they are flexible. Batch reactors are ideal for working with small and medium volumes, and the manufacturer can make a wide range of compounds with a high level of control.
2. Continuous Flow Reactors
So-called plug flow reactors are also becoming popular in high-volume production where quality and scale are of importance. They provide enhanced heat and mass transfer, lower reaction times, and can be used in automated systems.
3. Jacketed Reactors
Jacketed glass or stainless steel reactors are suitable for conducting exothermic or endothermic reactions because they are fitted with a jacket to circulate thermal fluids to transfer heat.
4. High-Pressure Reactors
High-pressure reactors (autoclaves) are necessary to perform processes that involve high pressures and temperatures, e.g., hydrogenation or catalytic reactions.
Key Factors in Pharmaceutical Reactor Design
The task of designing a pharmaceutical reactor is not limited to choosing the type of reactor, but it is to optimize several parameters to achieve operational, safety and regulatory needs.
1. Building Material
Depending on the chemical process and cleaning procedures, pharmaceutical reactors should be constructed out of non-reactive and GMP-compliant material, SS316L, glass-lined steel or Hastelloy.
2. Heat Transfer And Thermal Control
The reactions are usually exothermic or endothermic. Safety and uniformity of yield are achieved by proper thermal control with jackets or internal coils.
3. Agitation and Mixing
Appropriate agitation will avoid hot spots and guarantee the even dispersion of reactants. The choice of design elements, such as impellers, baffles and stirrers, is made on the basis of viscosity, phase mixing and reaction kinetics.
4. Scalability
Scaling-up, i.e., transfer of designs to larger-scale reactors, to pilot scale and full-scale production, must be possible without change of reaction pathway or quality loss.
5.Cleaning and Sterilisation
CIP and SIP systems are included to allow simple cleaning and sterilization to comply with cGMP, and to avoid cross-contamination in facilities that produce multiple products.
Challenges of Scaling Up Reactor Systems
When scaling a pharmaceutical reaction that is performed in a laboratory to commercial production, it is more than just an increase in volume. It brings in complex variables, which include:
- Heat and mass transfer loses
- Pressure drop, Flow rate discrepancies
- A threat of inconsistent batches
- Mechanical integrity under increased loads
Stalwart International addresses these challenges by offering custom-designed reactor systems that ensure identical process parameters during scale-up. Sophisticated simulation technologies and pilot tests enable the ease of transitions without compromising the integrity of the product.
Integration with Auxiliary Process Equipment
Pharmaceutical reactors are usually used with other devices, including:
- Controlled-temperature heat exchangers
- Solvent recovery and reflux systems, Condenser systems
- Filter presses to separate solid-liquid 6
- High-pressure storage or reaction pressure vessels
Stalwart specializes in providing integrated systems of pharmaceutical process equipment that can improve the efficiency of operations and minimize downtimes.
Compliance with cGMP and Global Standards
In the regulated industries, drug manufacturing is one of the examples where compliance is mandatory. All equipment should be compliant with current Good Manufacturing Practices (cGMP) and certifications such as ASME, ISO 9001 and FDA compatibility.
Stalwart International’s reactors are engineered with:
- Hygienic design concepts
- Welds without any breaks and mirror-polished finishing
- Documentation services (DQ, IQ, OQ, PQ)
- International standard certifications to make it easy to validate
You May Also Like: Importance of cGMP in the Pharmaceutical Industry
Case Study: API Manufacturing Scale-Up
A leading pharmaceutical company approached Stalwart International, a trusted pharmaceutical process equipment manufacturer, to scale their laboratory synthesis process for a high-value API. Initially conducted in a 20L glass reactor, the process needed to be scaled to a 2000L SS316L reactor while maintaining identical yields and reaction conditions.
Stalwart’s engineering team designed and built a customized jacketed reactor, complete with automated control panels, integrated heat exchangers, and CIP capabilities. The scale-up was validated through successful pilot trials, and full-scale production commenced without any quality deviations—demonstrating Stalwart’s excellence in delivering reliable and precise pharmaceutical equipment solutions.
Why Choose Stalwart International for Pharmaceutical Reactor Systems?
With decades of experience in process equipment manufacturing, Stalwart International delivers:
- Lab, pilot, and production scale pharmaceutical reactors custom-built
- Complete adherence to cGMP and ASME regulations
- Consolidation of auxiliary systems such as condensers, filters and pressure vessels
- Worldwide delivery and commissioning services
- Experience in the upscaling of high-precision pharmaceutical processes
You May Also Like: Why Stalwart’s Client-Centric Approach Wins
Final Thoughts
The scaling and designing of a pharmaceutical reactor system is a science as well as an art. It requires rich process knowledge, regulatory know-how and engineering skill. You do not want to be a first-time manufacturer when it comes to developing a new molecule or ramping up API production. When working with Stalwart International, you will partner with an experienced Industrial reactor manufacturer to make sure that your process is safe, scalable, and compliant.
FAQs
1. What is pharmaceutical reactor design?
Pharmaceutical reactor design refers to the engineering and configuration of vessels used to carry out chemical reactions under controlled conditions in pharmaceutical manufacturing. It involves selecting appropriate materials (like stainless steel or glass-lined), designing mixing systems, controlling temperature and pressure, and ensuring compliance with GMP standards. The goal is to optimize reaction efficiency, product purity, and safety.
2. How do you choose the right type of pharmaceutical reactor?
Choosing the right reactor depends on several key factors:
- Type of reaction (exothermic, endothermic, enzymatic)
- Batch vs continuous process
- Corrosiveness of reactants or solvents
- Scalability and production volume
- Regulatory compliance (e.g., FDA, GMP)
Common reactor types in pharma include Glass-Lined Reactors (GLRs), Stainless Steel Reactors (SSRs), Batch Reactors, and Continuous Stirred Tank Reactors (CSTRs).
3. What are the different types of reactors used in the pharmaceutical industry?
Pharmaceutical industries typically use the following reactor types:
- Batch Reactors – Ideal for multi-step processes and flexible production.
- CSTR (Continuous Stirred Tank Reactor) – Suitable for continuous flow reactions.
- PFR (Plug Flow Reactor) – Used in large-scale production where uniform product quality is needed.
- Glass-Lined Reactors (GLR) – For corrosive and heat-sensitive reactions.
- Stainless Steel Reactors (SSR) – Widely used for high-pressure or high-temperature reactions.
4. How do you size a pharmaceutical reactor?
Reactor sizing is based on:
- Reaction kinetics and residence time
- Volume of reactants and products
- Heat transfer requirements
- Agitation and mixing needs
The volume of the reactor (V) can be calculated using:
V = (Flow rate) × (Residence Time)
Further CFD modeling and scale-up studies ensure the design is efficient for production and safety.
5. Why is a glass-lined reactor (GLR) used in pharmaceuticals?
- GLRs are popular in pharma because they offer:
- Corrosion resistance to acids, alkalis, and solvents
- Non-reactive surface ensuring product purity
- Ease of cleaning and sterilization
- Compliance with FDA and GMP standards
GLRs are ideal for reactions involving sensitive or hazardous materials.